Eli Lilly’s GIP/GLP-1 dual-target agonist receives FDA approval

On Could 13, Eli Lilly introduced that the US FDA accredited the corporate’s glucose-dependent insulin-stimulating polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor twin agonist Mounjaro (tirzepatide) itemizing, weekly A single injection to complement weight loss program and train to enhance glycemic management in adults with sort 2 diabetes. Lilly mentioned Mounjaro represents the primary new sort of diabetes drug in almost a decade.

Mounjaro will probably be accessible in 6 doses (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg) and will probably be accessible in Lilly’s well-established auto-injector pen with a pre-attached hidden needle that the affected person can not contact or see not.
Tirzepatide is a weekly injectable GIP and GLP-1 receptor agonist that mixes the actions of two incretins into one new molecule. GIP is a hormone that enhances the motion of GLP-1 receptor agonists. Preclinical fashions have demonstrated that GIP reduces meals consumption and will increase vitality expenditure, leading to weight reduction. And when GIP is utilized in mixture with a GLP-1 receptor agonist, it could have a larger impact on affected person blood sugar and physique weight.
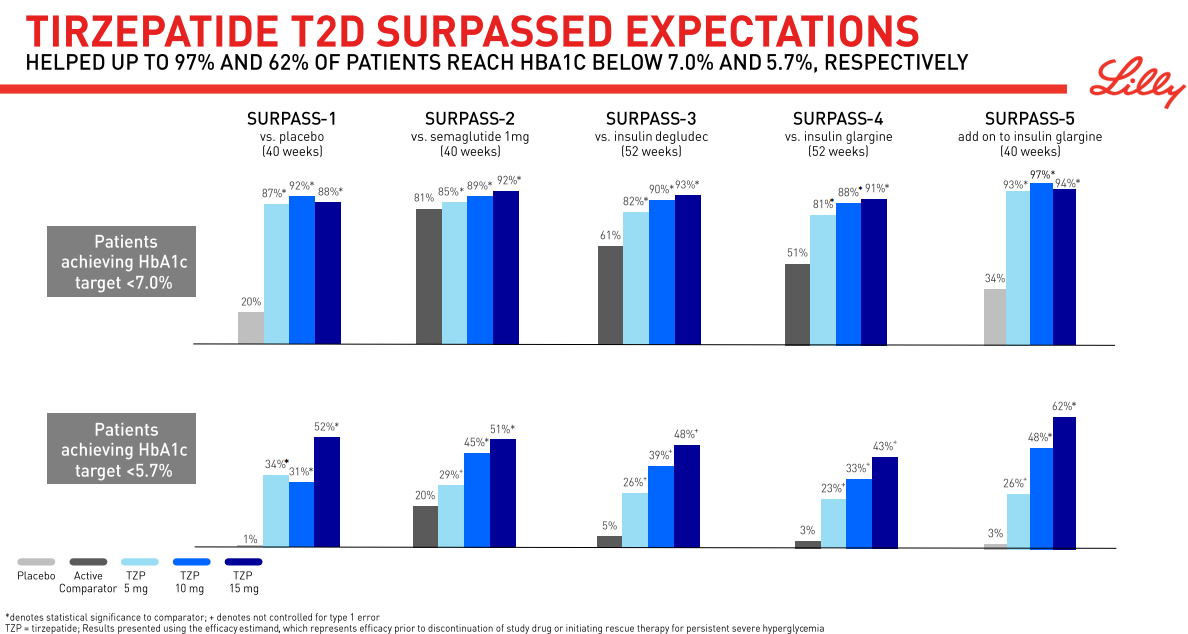
The approval relies on constructive outcomes from the Section III SURPASS program, a big Section III program consisting of 10 scientific trials deliberate to enroll greater than 13,000 sufferers with sort 2 diabetes, 5 of that are world registry research. These embody activity-controlled research with semaglutide 1 mg, insulin glargine, and insulin degludec. These research evaluated Mounjaro (5mg, 10mg and 15mg) alone or together with generally prescribed diabetes drugs together with metformin, SGLT2 inhibitors, sulfonylureas and insulin glargine. Within the SURPASS program, the 5mg dose of Mounjaro lowered topics’ A1C by a median of 1.8%-2.1%, and the 10mg and 15mg doses lowered topics’ A1C by a median of 1.7%-2.4%.
As well as, Tirzepatide has additionally achieved success in weight reduction. Lately, within the Section III SURMOUNT-1 trial, the load loss impact of tirzepatide (5mg, 10mg, 15mg) therapy group was considerably higher than the placebo management group at week 72 , the typical weight reduction reached a most of twenty-two.5% (24kg), and 63% of sufferers within the 15mg high-dose group misplaced greater than 20% of their weight. Lilly mentioned it would talk about with the FDA and search regulatory approval for this indication.